Biologic Drug Analytics
Cell-based Assays
ProtaGene offers state-of-the-art assays to evaluate the potency of your product for safety and efficacy.
Cell-based assays
- Reporter Gen Assays (RGA)
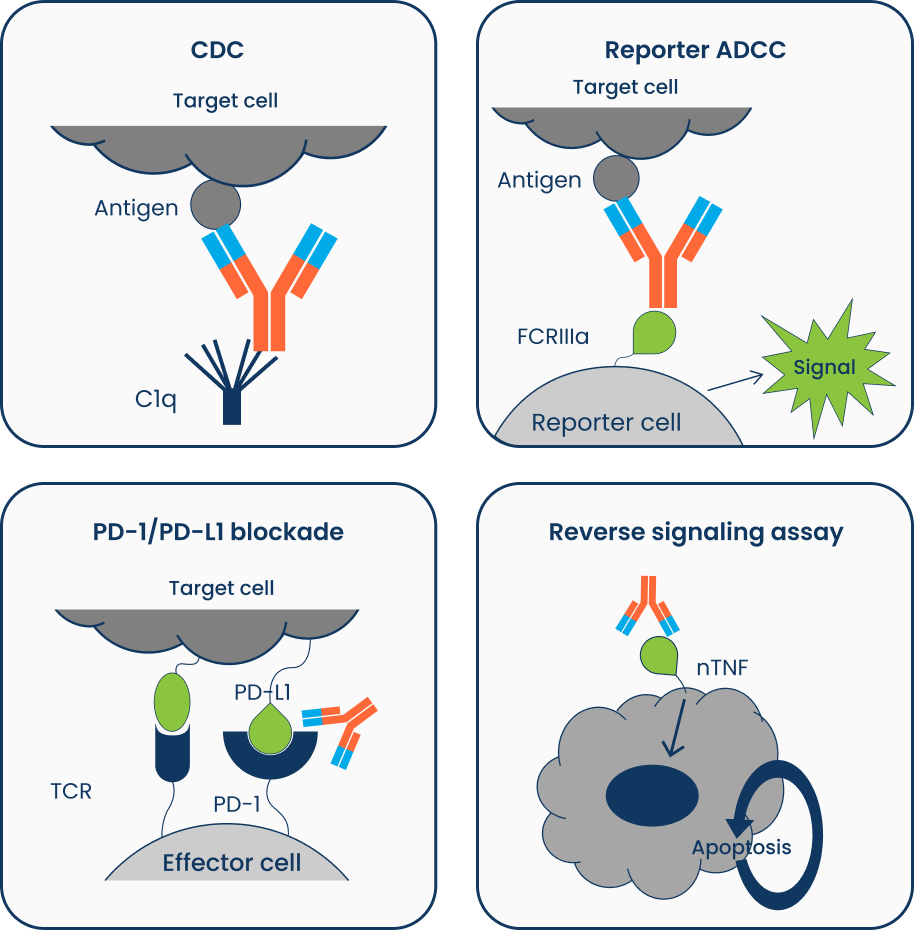
- Effector Function – Complement Dependent Cytotoxicity (CDC)/Antibody Dependent Cellular Cytotoxicity (ADCC)
- CDC: Does my mAb activate the complement system either on purpose (MoA) or as an unwanted side effect (safety & efficacy)?
- Reporter ADCC: Does my mAb bind to FCRIIIa receptor either on purpose (MoA) or as an unwanted side effect (safety & efficacy)?
- Does my mAb initiate apoptosis in cells expressing the target antigen?
Binding assays
-
SPR
-
ELISA
ProtaGene’s dedicated cell culture and cell-based assay team is built to support our partners with unique expertise and high capacity.
-
GMP-compliant structural characterization expertise to elucidate potential effects on your Molecule’s Potency (Structure-Function Correlation)
-
ProtaGene offers molecule-centered structural characterization with high-resolution mass spectrometry to evaluate effects on molecule potency
-
Utilization of bioluminescence-based read-outs enable robust signal detection
-
Establishment of thaw & use working cell banks will enable fast turnaround times


A centralized project management team to support your product at all project stages.
-
Highest flexibility in project planning and operations, including workflow adaptation, reporting, and alignment of QM-systems and validation parameters
-
bullet Created with Sketch. Close collaboration with partner to discuss project results and analytical strategies
-
bullet Created with Sketch. Internal and centralized project management as a single point of contact, including financial and operational topics
Analytical Solutions from Research to Market
Biologic Therapeutics Platform Expertise
Our deep experience advancing a diverse range of therapeutic platforms from research through market help the ProtaGene team design highly effective analytical programs that de-risk development and accelerate timelines to your next key milestones. Key biologic development areas include:
-
bullet Created with Sketch. Recombinant proteins—including complex, highly glycosylated proteins
-
bullet Created with Sketch. Multi-subunit complexes—protein, nucleotide, ligand
-
bullet Created with Sketch. mAbs
-
bullet Created with Sketch. ADCs and protein conjugates
-
bullet Created with Sketch. PEGylated proteins
-
bullet Created with Sketch. Bispecifics/multispecifics
-
bullet Created with Sketch. Fusion proteins
-
bullet Created with Sketch. Enzyme replacement therapies
-
bullet Created with Sketch. Biosimilars
-
bullet Created with Sketch. Vaccines, including subunit/recombinant
Analytical Systems
Enabled by state-of-the-art technology, ProtaGene offers an extensive range of analytical capabilities to support your biotherapeutic or gene & cell therapy projects.